HAWAIIAN CORAL REEFS
Cortney Cameron
EOS 402S, Duke University
I. Introduction
He pūko‘a kani ‘āina.
A coral reef grows into an island (from small beginnings come great things).
Part animal, part plant, and part mineral, corals sit at the crossroads of biology and geology. In the Hawaiian Islands, geographic isolation and hot spot volcanism have created a uniquely trying environment for coral reef development. This paper will summarize the biology and ecology of Hawaiian corals, as well as their economic and cultural importance, in order to understand the geologic contributions that corals make in producing carbonate and aiding the interpretation of igneous features.
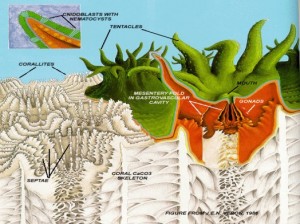 Figure 1. Coral polyp (green and red) on corallite (white). Inset: Cnidocyte with nematocyst. From Cohen & Holcomb, 2009. Figure 1. Coral polyp (green and red) on corallite (white). Inset: Cnidocyte with nematocyst. From Cohen & Holcomb, 2009. |
II. General Biology
A. Classification and Traits
Corals are metazoan colonial marine invertebrates belonging to the phylum Cnidaria (shared by jellyfish), class Anthozoa [Ruppert, Fox & Barnes, 2004]. Defining traits of cnidarians include radial symmetry, with a central mouth surrounded by tentacles sporting cnidocytes [Tardent, 1995]. Cnidocytes contain organelles called nematocysts (“stinging cells”) resembling coiled tubes; touch receptors cause water to rush into the nematocyst, which explodes and releases toxic spines that immobilize prey. Nematocysts on the tentacles are used to capture zooplankton; prey is pulled into the gut through the mouth, where gastrodermal nematocysts hold live prey in place during digestion [Ruppert, Fox & Barnes, 2004]. Septa dividing the gastrovascular cavity increase digestive surface area; waste is excreted back through the mouth.
 Figure 2. Porites sp.polyps (2 mm) in Turks and Caicos. Photo by C. Cameron. Figure 2. Porites sp.polyps (2 mm) in Turks and Caicos. Photo by C. Cameron. |
Carnivorous consumption serves as only one source of nutrition in calcifying corals of the order Sclerentina, which precipitate aragonite skeletal structures (see Section 3A) and represent the vast majority of reef-builders [Scoffin, 1987]. Scleractinians are obligate symbionts with photosynthetic dinoflagellate algae known as zooxanthellae [Fenner, 2005]. Zooxanthellae utilize wastes released by corals—such as carbon dioxide—to aid the host in building calcium carbonate at up to several millimeters per year [Jokiel, 1987]. In part due to their relationship with photosynthesizers, corals commonly inhabit shallow tropical seas [Scoffin, 1987]; this intolerance of cool, deep waters allows for reconstruction of sea surface temperature (SST) (see Section 3C).
Although individual polyps are only a few millimeters in height and diameter (Fig. 1), coral reef frameworks can grow so large as to become islands (see Section 3B). The corallite cups excreted by polyps join to form colonies called heads (Fig. 2), and many coral heads can combine to form reefs. Polyps reproduce asexually to grow individual coral heads, while new heads form by spawning free-swimming larvae [Jokiel, 1987].
| Table 1. Characteristics of phylum Cnidaria and class Anthozoa. | |
| Cnidarians | Anthozoans |
| Cnidocytes | Nematocysts lack operculum (hinged lid) |
| Radial symmetry | No medusa stage |
| Mouth, lack anus | Mesenteries (septa) divide gastrovascular cavity |
| Circle of tentacles around mouth | Mesoglea contains “wandering cells” |
B. Hawaiian Corals
 Figure 3. Melichthys niger (30 cm) swimming over Porites lobata mound in Kahalu’u Bay, Hawai‘i. Photo by C. Cameron. Figure 3. Melichthys niger (30 cm) swimming over Porites lobata mound in Kahalu’u Bay, Hawai‘i. Photo by C. Cameron. |
 Figure 4. Porites lobata (left) next to Pocillopora meandrina head (15 cm) in Kahalu’u Bay, Hawai‘i. Note coral rubble. Photo by C. Cameron. Figure 4. Porites lobata (left) next to Pocillopora meandrina head (15 cm) in Kahalu’u Bay, Hawai‘i. Note coral rubble. Photo by C. Cameron. |
The Hawaiian Islands, formed by the movement of the Pacific Plate over a stationary hot spot, extend almost 2,500 km from latitudes 19° N to 29° N, the latter representing the northernmost limit of coral growth (see Section 3B) [Grigg, 1982]. At 4,000 km from any large landmass, they are most geographically isolated islands in the world [Grigg, 1983; Frierson, 2012]. While this has resulted in extreme endemism on land (90% of species), speciation is not as drastic in the ocean due to its interconnectedness, also the reason coral species are uniform throughout the islands [Jokiel, 1987; Grigg, 2013]. Nevertheless, almost 20% of the 90 or so Hawaiian coral species are endemic to the islands, more than almost anywhere else in the world; of these, 42 species across 16 genera are calcifying reef-builders [Grigg, 1983; Jokiel, 1987; Fenner, 2005]. Relative to the number of species that could inhabit the Hawaiian Islands, however, this number is impoverished: the nearby Indo-West Pacific Ocean, the source of modern Hawaiian corals, contains 65 coral genera alone [Jokiel, 1987; Fletcher et al., 2008; Grigg, 2013].
Hawaii experiences two seasons: a summer characterized by an average SST of 27°C and consistent 40 km/h northeasterly trade winds, and a winter characterized by an average SST of 24°C and southerly Kona winds that bring storm events [Fletcher et al., 2008]. Decadally, powerful winter El Niño swells limit coral growth by destroying even the hardier corals that can withstand strong annual wave energy, a phenomenon that has suppressed reef building for the past 5,000 years and periodically clears half the Oahu shelf of live coral [Fletcher et al., 2008]. This physical disturbance acts at the largest control on coral reef structure in the Hawaiian Islands, and less than four species represent 75% of reef coverage [Fletcher et al., 2008].
 Figure 5. Halimeda sp. in Southern Florida. Photos by C. Cameron. Figure 5. Halimeda sp. in Southern Florida. Photos by C. Cameron. |
 Figure 6. Nerita picea (1-3 cm) on shoreline of Punalu’u Beach, Hawai‘i. Photo by C. Cameron. Figure 6. Nerita picea (1-3 cm) on shoreline of Punalu’u Beach, Hawai‘i. Photo by C. Cameron. |
In shallower exposed settings, the dominant reef-building species is the wave-tolerant Porites lobata (lobe coral), which exhibits two forms—encrusting and massive—depending on prevailing energy conditions [Grigg, 1983; Fletcher et al., 2008]. Massive P. lobata occur in lower-stress reef flats andbuild yellow lumpy mounds up to 6 m across (Fig. 3) [Fenner, 2005]. Pocillopora meandrina (cauliflower coral) is another common Hawaiian coral, forming bumpy cream-colored branches up to 25 cm long (Fig. 4) [Fenner, 2005]. As a pioneer species, P. meandrina quickly colonizes lava flows [Grigg, 1983]. In deeper protected waters, Porites compressa (finger coral) prevails. In March 2014 at Kahalu’u Bay on Hawai‘i, the author observed coverage as 85% P. lobata, 10% P. meandrina, and 5% Pavona varians (corrugated coral).
Other important carbonate-producing species in Hawai‘i include Halimeda (a green algae which also plays a top roles in Caribbean sediment production), Porolithon (a red algae), molluscs, and foraminifera (Figs. 5 & 6) [Moberly & Walker, 1987; Blay, 1999; Fletcher et al., 2008].
III. Geologic Contributions
A. Calcium Carbonates
The two carbonate minerals precipitated by calcifying marine organisms are the polymorphs aragonite and calcite, which have the CO3 group as their base [Scoffin, 1987]. The CO3 unit resembles a triangle with the carbon atom in the center and the oxygen atoms at the corners. The calcium cation interacts with this group to produce an orthorhombic structure in aragonite and a trigonal one in calcite. Both minerals present as colorless or white in hand sample with cleavage along three planes. The less stable of the two forms, aragonite will often recrystallize into calcite, which can complicate fossil studies.
| Table 2. Contrasting properties of aragonite and calcite (after Scoffin, 1987). | ||
| Aragonite | Calcite | |
| Crystal System | Orthorhombic | Trigonal |
| Indicatrix | Biaxial Negative | Uniaxial Negative |
| Hardness | 3.5-4 | 3 |
| Specific Gravity | 2.94 | 2.72 |
Calcium carbonates react reversibly with carbonic acid (formed by carbon dioxide and water) to produce calcium and bicarbonate:
CaCO3 + CO2 + H2O ⇌ Ca + 2HCO3
In the ocean, beneath the carbonate compensation depth—which is controlled by seawater temperature, pressure, and chemical composition—the reaction skews to the right such that no carbonates are preserved. Above this depth, calcium carbonate can exist as solid skeletal material and sediments; currently in the Pacific Ocean, this depth is 4.6 km [Pälike et al., 2012]. Although aragonite is harder than calcite (Table 2), aragonite is more soluble and so has a shallower compensation depth. This raises concerns about the ability of corals, which excrete aragonite (Fig. 7), to cope with anthropogenic ocean acidification [Orr et al., 2005].
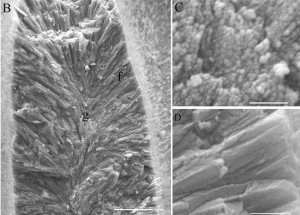 Figure 7. Aragonite crystals precipitated by coral: granular during nighttime (g, C) and needlelike during daytime (f, D). Scalebars: B, 10 μm; C and D, 1 μm. From Cohen & Holcomb, 2009. Figure 7. Aragonite crystals precipitated by coral: granular during nighttime (g, C) and needlelike during daytime (f, D). Scalebars: B, 10 μm; C and D, 1 μm. From Cohen & Holcomb, 2009. |
Like most marine calcifiers, corals precipitate calcium carbonate by elevating their pH relative to ambient seawater; however, instead of using specialized internal precipitation compartments, corals sit atop their skeleton and precipitate aragonitic needles directly externally [Cohen & Holcomb, 2009]. During this process, corals incorporate magnesium—which is readily available in seawater—into the skeletal lattice, with the ratio of magnesium to calcium increasing with increasing temperature; when combined with oxygen isotope data and coral growth rates, the Mg/Ca relationship can be used to reconstruct past SSTs [Watanabe, Winter, & Oba, 2001]. Inorganic calcium carbonates do not contain magnesium in the lattice [Scoffin, 1987].
B. Land-forming: Atolls and Other Contributions
 Figure 8. Fringing reefs around Diamond Head, Oahu. Photo by C. Cameron. Figure 8. Fringing reefs around Diamond Head, Oahu. Photo by C. Cameron. |
In the Hawaiian Islands, where the formation of land is dominated by igneous processes that also disrupt coral growth, corals do not represent an important part of island-building like they do in Florida and the Caribbean. However, after volcanism ceases, basalts provide the required hard substrate for the colonization of corals, which can then reach full successional climax in roughly 50 years [Grigg, 1983].
In 1845, Charles Darwin penned one of the earliest known theories of atoll formation, which, with some modifications, remains accepted today [Darwin, 1915; Grigg, 1982; Fletcher et al., 2008]. As the northward movement of the Pacific Plate carries islands away from heat and magma supplied by the hotspot, eruptions stop and subsidence begins [Grigg, 1982; Grigg, 1997]. No longer threatened by lava flows, corals quickly colonize the substrate; fringing reefs develop along the edges of the dying volcano (Fig. 8), eventually completely encircling the rim as a barrier reef. Once the volcano sinks below sea level, only the ring of coral—now called an atoll—remains near the surface (Figs. 9 & 10).
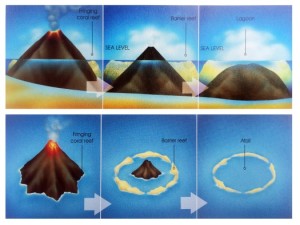 Figure 9. Atoll formation in Hawaiian Islands. From Grigg 2013. Figure 9. Atoll formation in Hawaiian Islands. From Grigg 2013. |
In the Hawaiian Islands, fringing reefs can develop as in as little as 100 years, barrier reefs in two million, and atolls in ten million [Grigg, 1997]. By comparison, the Society Islands in the South Pacific have atolls as young as four million years; slower atoll development in the Hawaiian Islands is attributed to their average 2 km greater initial elevation and the presence of just one fourth as many coral species [Grigg, 1997].
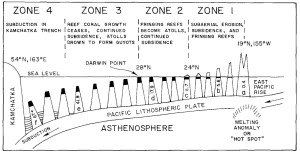 Figure 10. Formation of guyots. Age of islands in millions of years for a-f. From Grigg 1982. Figure 10. Formation of guyots. Age of islands in millions of years for a-f. From Grigg 1982. |
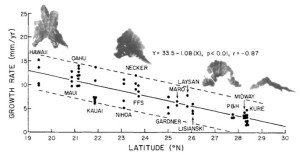
As long as the vertical accretion of corals keeps pace with subsidence, the atoll persists. Coral growth rates vary between 1-10 mm per year depending on species, temperature, and available light, with the record of 15 mm per year held by the Caribbean Acropora palmata [Grigg, 1997]. In the Hawaiian Islands, as tectonic movement carries the atolls northward, cooler temperatures inhibit growth; in fact, coral accretion decreases linearly as a function of latitude (Fig. 11). At the Darwin Point, which today stands at 29° N, reef growth ceases entirely and the reef drowns, creating seamounts and flat-topped guyots [Grigg, 1982]. Since coral species are uniform through the islands (see Section 2B), growth rates can be compared directly; the common P. lobata accretes at 13 mm per year near the Big Island, but slows down to less than 3 mm per year at the extreme northeastern end of the chain [Grigg, 1982].
When sea level drops, reefs can become limestone islands, as seen in Florida and the Caribbean, and to a lesser extent, the Northwestern Hawaiian Islands. Key Largo in the upper Florida Keys is famous for its coralline limestone (Fig. 12). In the Hawaiian Islands, however, subsidence negates much of the progress of reef-builders, and the coral islands in the northwest are small and devoid of people. One noteworthy super-island called Maui-Nui formed 21 thousand years ago, when sea level dropped 120 m to expose limestone bridges connecting Maui, Lanai, and Molokai [Fletcher et al., 2008; Grigg, 2013].
 Figure 12. Brain coral in Key Largo Limestone in Florida. Photo by C. Cameron. Figure 12. Brain coral in Key Largo Limestone in Florida. Photo by C. Cameron. |
Although they might not feature a star role in island-building in Hawai‘i, corals do play an important part in generating sediment. As reef-builders die or are broken apart by wave action and bio-erosion, they create sediment [Fletcher et al., 2008]. The 12 km2 reef in Kailua Bay in Oahu produces an estimated 4,000 m3 of calcareous sediment per year [Fletcher et al., 2008]. In fact, any white beach in Hawaii owes its existence to carbonate producers, as basalts create black-to-brown sediments and Hawai‘i has no significant natural source of quartz. The longer an island has lacked volcanism, the higher the percentage of carbonate on its beaches; even on the northeastern side of the Big Island, which is only relatively recently inactive, coral fragments dominant beach sand [Blay, 1999].
This carbonate sand can eventually lead to the formation of beachrock, a limestone that precipitates at shorelines due to the interaction of calcareous sediment and groundwater (Fig. 13). Although beachrock forms inorganically, corals and other calcifiers have helped by making the calcium carbonate available [Emery & Cox, 1956].
 Figure 13. Beachrock overlying basalt at Kokololio Beach, Oahu. Photo by C. Cameron. Figure 13. Beachrock overlying basalt at Kokololio Beach, Oahu. Photo by C. Cameron. |
C. Dating Igneous Features
Due to the limited environmental ranges they can inhabit and their known growth rates (see Section 2A), corals can aid in interpreting igneous features. Reefs drown not only in response to lowered temperatures, but also in response to subsidence or rising sea level, as deeper waters prevent the penetration of light required for zooxanthellae photosynthesis. Similarly, reefs will die if they are subaerially exposed by uplift or dropping sea levels.
 Figure 14. Coral fragments in tuff at Hanauma Bay. Photo by C. Cameron. Figure 14. Coral fragments in tuff at Hanauma Bay. Photo by C. Cameron. |
In the Hawaiian Islands, land masses begin subsiding as soon as they move off of the hot spot. On the Big Island, subsidence is fastest near the active southern coast and slowest near the north; this differential subsidence tilts coral reefs toward the island [Moore, 1987]. Dating tilted reefs off of the Big Island has provided information on subsidence rates and the timing of eruption cessation [Campbell, 1984; Moore, 1987]. Near Kohala to the north, drowned reefs indicate subsidence of 2.6 mm per year over the past 475,000 years [Campbell, 1984; Szabo, Moore, & Simmons, 1991]. Moreover, as the weight of the Big Island depresses the lithosphere, rebound causes a compensatory bulge around the depression, uplifting Oahu at 0.06 mm per year over the same half million year time span, indicated by elevated corals in the growth position [McMurty et al., 2010; Grigg, 2013].
The presence of corals in deposits also provides insight into depositional envrionments. In Hanauma Bay, coral fragments in tuff suggest that subaqueous eruptions created the deposits (Fig. 14). Ash at the base of cores drilled through the reef in here show that eruptions preceded coral growth, while the age of the first corals reveal when water flooded the bay [Easton & Olson, 1976]. Finally, as discussed in Section 3C, sand composition can provide relative age information (Fig. 15); by the older northern Midway Atolls, volcanic fragments comprise virtually none of sand [Blay, 1999].
IV. Importance
A. Ecological & Economic Services
Coral reefs provide at least 26 distinct services to people, including as renewable resources for medicine, cement, and seafood; worldwide, reefs support a third of all marine fish species (Fig. 16) and supply 10% of fish mass consumed by humans [Moberg & Folke, 1999]. In Hawai‘i, reefs generate a minimum of $364 million in economic stimulus each year, including $304 million in recreational value and $40 million in property value [Bishop et al., 2011]. For tourists partaking in reef-related activities such as diving and fishing, an average of $184 per visit is spent—not counting related food and lodging expenditures [Brander, Beukering, & Cesar, 2007].
 Figure 16. Chlorurus spilurus (30 cm) feeding on coral in Kahalu’u Bay, Hawai‘i. Photo by C. Cameron. Figure 16. Chlorurus spilurus (30 cm) feeding on coral in Kahalu’u Bay, Hawai‘i. Photo by C. Cameron. |
Ecologically, coral reefs protect coastal ecosystems (and properties) by buffering currents and waves, as well as generating sediment at up to 1,000 g CaCO3 per m2 per year [Chave, Smith, & Roy, 1972; Moberg & Folke, 1999]. Furthermore, corals fix nitrogen and over geologic time scales, act as net sinks of carbon dioxide [Moberg & Folke, 1999]; the indigenous Hawaiian species Pocillopora damicornis fixes up to 29 g of carbon per m2 per day, one of the highest known primary production rates [Jokiel, 1987]. Finally, corals provide information services by recording climate (see Section 3A) and pollution levels [Moberg & Folke, 1999].
B. Cultural Importance
Corals feature in numerous native Hawaiian myths. In the Hawaiian creation chant, Kumulipo, corals form on the first night to later give rise to all other organisms, underscoring the importance of corals as ancestral in the Hawaiian worldview [Frierson, 2012]. Accordingly, Kāne, creator of life and the highest of the four major Hawaiian deities, was associated with coral [Beckwith, 1970].
Similarly, corals play a major role in the formation of the Hawaiian islands. In one story, after catching pieces of coral, a legendary fisherman utters a prayer and tosses them into the ocean, where they grow into the major islands [Beckwith, 1970].
A land found in the ocean,
Thrown up out of the sea,
From the very depths of Kanaloa,
The white coral in the watery caves
That caught on the hook of the fisherman…
In another tale, the goddess of coral, who can take the form of a woman or a reef, gives the great demigod Maui a hook fashioned out of shell; Maui uses this hook to drag the major islands closer together, leaving footprints in the reefs of each island as he worked [Beckwith, 1970].
In addition to the spiritual importance of corals, native Hawaiians made practical uses of corals [Titcomb et al., 1978]. Antipathes spp. (black coral) were used extensively in jewelry and in medicine. One treatment for sores around children’s mouths involved applying a paste of powdered coral, decaying banana tree juice, mountain apple bark, and nuts. Various lung problems were treated by drinking a liquid of powdered coral, onions, flowers, and sugarcane mixed into coconut milk. Hard corals, such as Porites spp., were used as sandpaper to polish canoes and remove bristles from pigs.
V. Conclusion
Although corals do not form appreciable land masses in Hawaii as they do in the Caribbean, they create atolls, contribute sediments to beaches, and record paleoclimate. As the ocean becomes more acidic, their role as dominant sediment producers will likely be overtaken by more resistant organisms, such as foraminifera and molluscs. Even so, the economic and cultural importance of coral reefs is irreplaceable. In the face of threats including trampling and pollution, research and conservation efforts will continue to be important in understanding and preserving coral reefs [Hunter & Evans, 1995].
VI. References
Beckwith, M. W. (1970). Hawaiian Mythology. University of Hawai‘i Press: Honolulu.
Bishop, R. C., D .J. Chapman, B. J. Kanninen, J. A. Krosnick, B. Leeworthy, and N. F. Meade. (2011). Total Economic Value for Protecting and Restoring Hawaiian Coral Reef Ecosystems: Final Report. Silver Spring, MD: NOAA Office of National Marine Sanctuaries, Office of Response and Restoration, and Coral Reef Conservation Program. NOAA Technical Memorandum CRCP 16.
Blay, C. T. (1999). Compositional analysis of Hawaiian beach sediment: an indicator of source, process and coastal change. The Non-Steady State of the Inner Shelf and Shoreline: Coastal Change on the Time Scale of Decades to Millennia, University of Hawai‘i: Honolulu.
Brander, L. M., Van Beukering, P., & Cesar, H. S. (2007). The recreational value of coral reefs: a meta-analysis. Ecological Economics, 63(1):209-218.
Campbell, J. F. (1984). Rapid subsidence of Kohala Volcano and its effect on coral reef growth. Geo-marine Letters, 4(1), 31-36.
Chave, K. E., Smith, S. V., & Roy, K. J. (1972). Carbonate production by coral reefs. Marine Geology, 12(2), 123-140.
Cohen, A. L., & Holcomb, M. (2009). Why corals care about ocean acidification: uncovering the mechanism. Oceanography, 22(4):118-127.
Darwin, C. (1915). Works of Charles Darwin: Journal of researches into the natural history and geology of the countries visited during the voyage of HMS Beagle round the world (Vol. 1). D. Appleton and Company: New York.
Emery, K. O., & Cox, D. C. (1956). Beachrock in the Hawaiian islands. Pacific Science, X:382-402.
Easton, W. H., & Olson, E. A. (1976). Radiocarbon profile of Hanauma Reef, Oahu, Hawaii. Geological Society of America Bulletin, 87(5):711-719.
Fenner, D. (2005). Corals of Hawai‘i: A Field Guide to the Hard, Black and Soft Corals of Hawai‘i and the Northwest Hawaiian Islands, Including Midway. Mutual Publishing: Honolulu.
Fletcher, C. H., Bochicchio, C., Conger, C. L., Engels, M. S., Feirstein, E. J., Frazer, N., ... & Vitousek, S. (2008). Geology of Hawaii reefs. In Coral Reefs of the USA, 435-487. Springer Netherlands.
Frierson, P. (2012). The Burning Island: Myth and History in Volcano Country, Hawaii. Trinity University Press: San Antonio. p82
Grigg, R. W. (1982). Darwin Point: a threshold for atoll formation. Coral Reefs, 1(1):29-34.
Grigg, R. W. (1983). Community structure, succession and development of coral reefs in Hawaii. Marine Ecology Progress Series, 11:1-14.
Grigg, R. W. (1997). Paleoceanography of coral reefs in the Hawaiian-Emperor Chain–revisited. Coral Reefs, 16(1):S33-S38.
Grigg, R. W. (2013). In the Beginning: Archipelago: The Origin and Discovery of the Hawaiian Islands. Island Heritage Publishing: Waipahu, Hawaii.
Moberly, R & Walker, P. L. (1987). Coastal and volcanic geology of the Hanauma Bay area, Oahu, Hawaii. Cordilleran Section of the Geological Society of America: Decade of North American Geology, Centennial Field Guide Volume 1, 1:5.
Hunter, C. L., & Evans, C. W. (1995). Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bulletin of Marine Science, 57(2):501-515.
Jokiel, P. L. (1987). Ecology, biogeography and evolution of corals in Hawaii. Trends in Ecology & Evolution, 2(7):179-182.
Ludwig, K. R., Szabo, B. J., Moore, J. G., & Simmons, K. R. (1991). Crustal subsidence rate off Hawaii determined from 234U/238U ages of drowned coral reefs. Geology, 19(2):171-174.
McMurtry, G. M., Campbell, J. F., Fryer, G. J., & Fietzke, J. (2010). Uplift of Oahu, Hawaii, during the past 500 ky as recorded by elevated reef deposits. Geology, 38(1):27-30.
Moberg, F., & Folke, C. (1999). Ecological goods and services of coral reef ecosystems. Ecological Economics, 29(2):215-233.
Moore, J. G., & Campbell, J. F. (1987). Age of tilted reefs, Hawaii. Journal of Geophysical Research: Solid Earth (1978–2012), 92(B3):2641-2646.
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., ... & Yool, A. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437(7059):681-686.
Pälike, H., Lyle, M. W., Nishi, H., Raffi, I., Ridgwell, A., Gamage, K., ... & Sawada, K. (2012). A Cenozoic record of the equatorial Pacific carbonate compensation depth. Nature, 488(7413):609-614.
Pukui, M. K. (1983). ‘Ōlelo No‘eau: Hawaiian Proverbs & Poetical Sayings. Bishop Museum Press: Honolulu.
Ruppert, E. E., Fox, R. S., & Barnes, R. D. (2004). Invertebrate Zoology: A Functional Evolutionary Approach. 7th ed. Brooks/Cole: Belmont, CA.
Scoffin, T. P. (1987). An Introduction to Carbonate Sediments and Rocks. Chapman and Hall: New York.
Tardent, P. (1995). The cnidarian cnidocyte, a hightech cellular weaponry. Bioessays, 17: 351-362.
Titcomb, M., Fellows, D. B., Pukui, M. K., & Devaney, D. M. (1978). Native use of marine invertebrates in old Hawaii. Pacific Science, 32(4):325-386.
Watanabe, T., Winter, A., & Oba, T. (2001). Seasonal changes in sea surface temperature and salinity during the Little Ice Age in the Caribbean Sea deduced from Mg/Ca and 18O 16O ratios in corals. Marine Geology, 173(1):21-35.